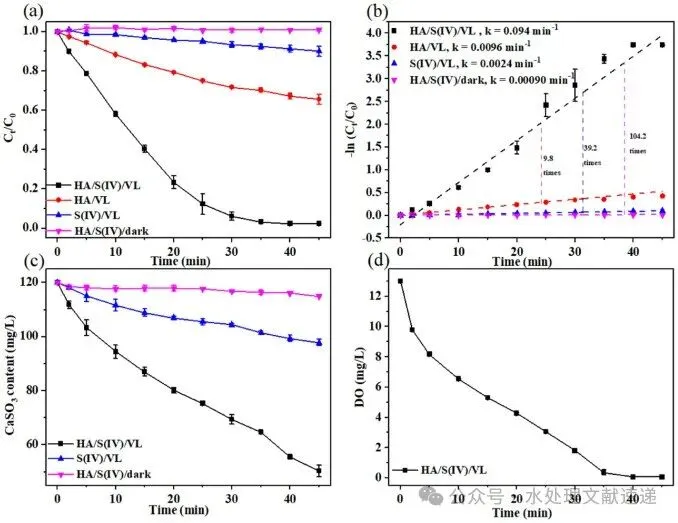
Fig. 1. The variation in ROX degradation percentage across different systems (a) and the corresponding pseudo-second-order kinetic fittings (b); the consumption kinetics of S(IV) across different systems (c); and the variation of dissolved oxygen in the S(IV)/HA/VL system (d). Experimental conditions: [S(IV)] = 1 mM, HA = 5 mg C/L, [ROX] = 5 mg/L, [initial pH] = 6.0 ± 0.05, T = 25 °C.
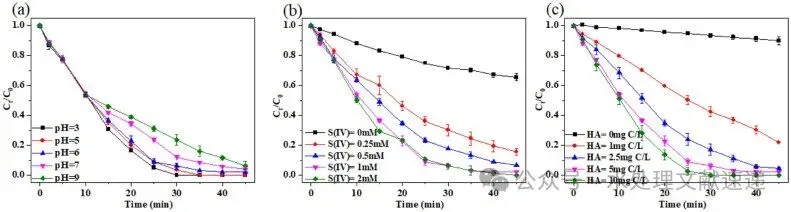
Fig. 2. Effects of operating parameters on ROX degradation in S(IV)/HA/VL system. (a) initial pH; (b) S(IV) concentration; (c) HA concentration. Experimental conditions: [ROX] = 5 mg/L, [HA] = 1 – 10 mg C/L, [S(IV)] = 0.25 – 2 mM, [initial pH] = 3.0 – 9.0, T = 25 °C.
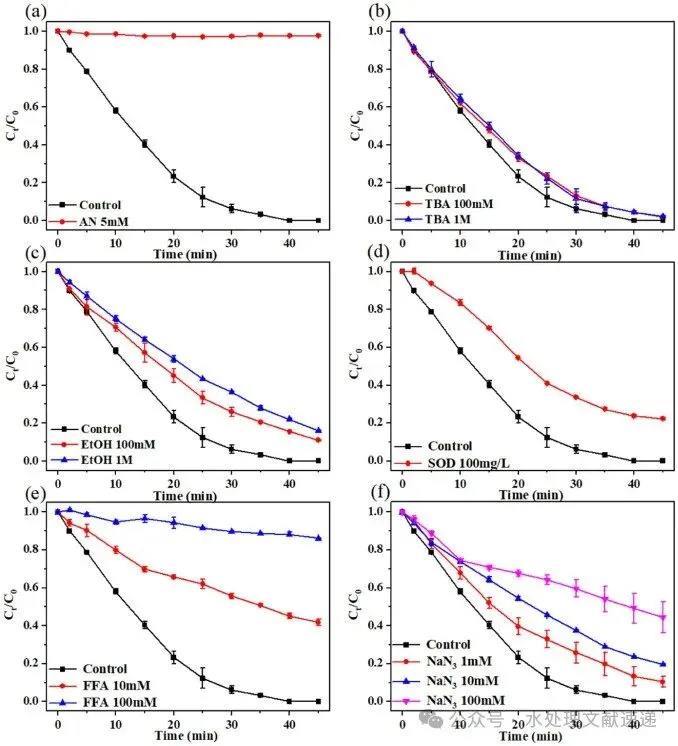
Fig. 3. Effect of AN(a), TBA (b), EtOH (c), SOD (d), FFA (e) and NaN3 (f) on ROX degradation in the S(IV)/HA/VL system. Experimental conditions: [ROX] = 5 mg/L, [S(IV)] = 1 mM, [HA] = 5 mg C/L, [initial pH] = 6.0 ± 0.05, T = 25 °C.
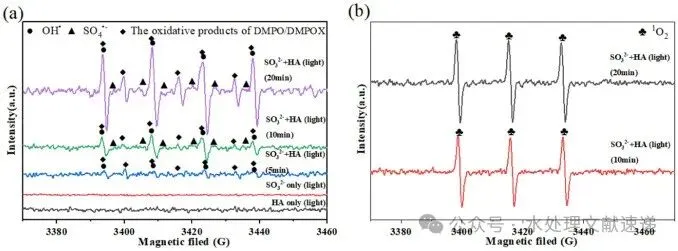
Fig. 4. DMPO (a) and TEMP (b) spin-trapping EPR spectra of HA /S(IV) /VL system. Experimental conditions: [S(IV)] = 1 mM, [HA] = 5 mg C/L, [initial pH] = 6.0 ± 0.05, T = 25 °C.
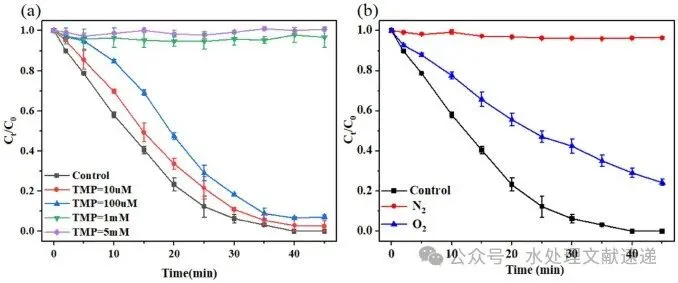
Fig. 5. Effect of different atmospheric conditions (a) and TMP (b) on the oxidation of ROX. Experimental conditions: [ROX] = 5 mg/L, [S(IV)] = 1 mM, [HA] = 5 mg C/L, [initial pH] = 6.0 ± 0.05, T = 25 °C.
Fig. 6. Effects of inorganic ions on ROX degradation (a) and the removal rate of ROX (b). Experimental conditions:(a) [ROX] = 5 mg/L, [S(IV)] = 1 mM, [HA] = 5 mg C/L, [initial pH] = 6.0 ± 0.05, inorganic ions = 5 mmol L−1, T = 25 °C;(b) [ROX] = 5 mg/L, [S(IV)] = 1 mM, [HA] = 5 mg C/L, [initial pH] = 6.0 ± 0.05, inorganic ions = 0 - 20 mmol L−1, T = 25 °C.